
The Unit is involved in all the computational work (modeling and simulation) needed during the stages of discovery-development and application of pharmaceutical products into clinical practice.
-
computational methodologies at the molecular level (e.g., drug – receptor interaction) which are required when searching for a new active substance,
-
quantitative structure relationships between molecular properties (e.g., MW, surface area) or physicochemical characteristics (e.g., solubility) and drug action or transit through the body (absorption, distribution, metabolism) characteristics,
-
computational design for development of the drug dosage form e.g., set specific release or dissolution properties, and the development of in vitro-in vivo correlations (IVIVC),
-
computational methods in pharmacokinetics and pharmacodynamics using non-compartmental or compartmental methods, as well as population approaches relying on nonlinear mixed effect models,
-
the mathematical and statistical analysis for the design and assessment of clinical and bioequivalence studies,
-
the application of population techniques based either on Bayesian approximations or control theory for determination, for setting the appropriate dosage regimen for narrow therapeutic index drugs,
-
all kinds of meta-analyses regarding drug action, side-effects, interactions, etc., in clinical use of drugs,
-
the use of bio-informatics in the drug design of biotechnology drugs.
-
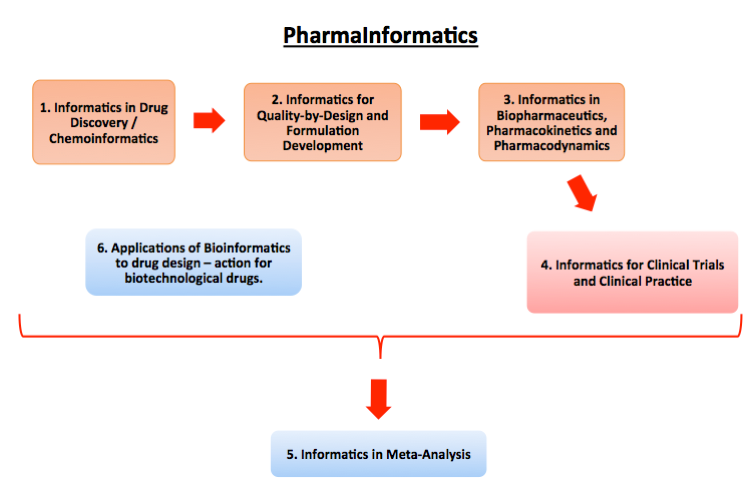
Informatics for Drug Discovery / Chemoinformatics
-
Informatics for Quality-by-Design and Formulation Development
-
Informatics for Biopharmaceutics, Pharmacokinetics, and Pharmacodynamics
-
Informatics for Clinical Trials and Clinical Practice
-
Informatics for Meta-Analysis
-
Applications of Bioinformatics to drug design-action of biotechnology drugs.
-
Panos Macheras: Professor Emeritus, Dpt. of Pharmacy, National and Kapodistrian University of Athens, Greece.
-
Emmanuel Mikros: Professor, Dpt. of Pharmacy, National and Kapodistrian University of Athens, Greece.
-
Haralambos Sarimveis: Professor, School of Chemical Engineering, National Technical University of Athens, Greece.
-
Aris Dokoumetzidis: Assistant Professor, Dpt. of Pharmacy, National and Kapodistrian University of Athens, Greece.
-
Evangelos Evangelou: Assistant Professor, Clinical and Molecular Epidemiology at University of Ioannina, Greece.
-
Kosmas Kosmidis: Dpt of Physics, Aristotle University of Thessaloniki, Greece
-
Ioannis Vizirianakis Associate Professor, Aristotle University of Thessaloniki, Greece
-
Dimitris Goussis, Professor, School of Applied Mathematics and Physical Sciences, National Technical University of Athens, Greece
-
Theodoros Christopoulos, Professor, Dpt. of Chemistry, University of Patras, Greece
-
Athanasios A. Tsekouras, Associate Professor, Department of Chemistry, Laboratory of Physical Chemistry, National and Kapodistrian University of Athens, Athens, Greece
-
Demeter Tzeli, Associate Professor, Department of Chemistry, Laboratory of Physical Chemistry, National and Kapodistrian University of Athens, Athens, Greece
In the recent years Pharma-Informatics Unit has evolved to focus primarily in the following four areas:
Modelling and Simulation in Biopharmaceutics, Pharmacokinetics, Pharmacodynamics Group
The founder of the Laboratory of Biopharmaceutics-Pharmacokinetics, University of Athens and founder of the PharmaInformatics Unit at ATHENA Research Center, Emeritus Professor Panos Macheras has carried out extensive research during his career in all above areas (see https://link.springer.com/journal/10928/46/2). For example, the adoption by EMA of the bioequivalence limits for highly variable drugs is associated with his work “On the leveling-off properties of the new bioequivalence limits for highly variable drugs of the EMA guideline. Eur. J. Pharm. Sci. 44, 497-505 (2011) 10.1016/j.ejps.2011.09.008”. During the last three years Panos has developed together with Athanasios Tsekouras, Associate Professor, National and Kapodistrian University of Athens, the Finite Absorption Time (FAT) concept. This work leads to a re-writing of oral pharmacokinetics and has serious implications for bioavailability/bioequivalence and biowaivers issues. Interested scientists or postgraduate students can find more on the current activities of the group at http://jupiter.chem.uoa.gr/pchem/lab/bppg.html.
Drug Design Group
The research group focuses on the rationalization of the mechanisms underlying potency and selectivity of New Molecular Entities against different pharmacological targets and their exploration for the discovery and targeted optimization of compounds as leads, chemical probes, or clinical candidate drugs. A number of in silico methods have been established and are actually in use in the lab (Molecular Mechanics-based docking-scoring calculations, FEP calculations, solvent mapping, Molecular Dynamics). We have recently proposed a specific protocol for virtual screening through an orthogonal consensus ranking combining 2D, 3D similarity and docking (Methods Mol Biol. 2018;1824:261-277, Future Med Chem. 2018;10:2411-2430). Important contributions have been achieved in the design of a considerable number of active molecules through the systematic study of pharmacological targets and more specifically a) Kinases: CDKs, GSK3, DYRK, CK1, Aurora (J. Med. Chem. 2004;47:935-946, J. Med. Chem. 2007;50:4027-4037, ACS Med. Chem. Lett. 2013;4:22-26, J. Nat Prod 2016;79:2464-2471 Int. J. Mol Sci, 2017;18:2102 b) Nuclear Receptors ERα, ERβ (Chem. Biol. 2004;11:397-406, Front. Chem. 2017;5:71. c) Epigenetic targets: bromodomains, UHRF1 (Eur J Med Chem, 2016;114:390-396, J. Med. Chem. 2016;59:8787-8803). Please address inquiries to: Emmanuel Mikros (Professor of Pharmaceutical Chemistry National & Kapodistrian University of Athens) (mikros@pharm.uoa.gr)
Machine Learning Group
Computational models that predict physicochemical properties, biokinetics or adverse biological effects of drug candidates are becoming increasingly important to support in-silico drug design. This is primarily due to cost-saving and reduction of attrition rates, since market candidates can be assessed early-on in the development process. Drugs predicted to be toxic or not possessing the required properties can be discarded before a significant amount of effort has been invested, and more significantly, before time-consuming and expensive experimental tests have been carried out. The group is developing computational pipelines that support the process of in-silico drug development, which include electronic representations of molecules, development of knowledge graphs, feature engineering, variable selection, machine learning and deep learning algorithms and optimal tuning of hyperparameters. Please address inquiries to: Haralambos Sarimveis (Professor of the School of Chemical Engineering of the National Technical University of Athens) (hsarimv@central.ntua.gr )
Pharmacometrics Group
Pharmacometrics is the discipline that studies by Modelling and Simulation approaches the pharmacokinetics and pharmacodynamics of drugs, and has the ultimate scope to build in silico clinical trials that can complement or even replace the real clinical trials. It has an impact throughout the entire drug development processes and is endorsed by the Regulatory Agencies in the context of what has been described as Model Informed Drug Development (MIDD). In the PMX Group of Pharma-Informatics we carry out methodological research as well as applications and for the latter we collaborate with the pharmaceutical industry, hospitals and international consortia. Emphasis is given to Physiologically Based Pharmacokinetic models (PBPK) and Population Pharmacokinetics / Pharmacodynamics models. Please see the projects section for details on specific projects. Also visit www.pharmacometrics.gr for more details. Please address inquiries to: Aris Dokoumetzidis (Associate Professor of the School of Pharmacy of the University of Athens) (adokoum@pharm.uoa.gr)